
The market for repeat expansion disorders treatment is expected to grow at a CAGR of 13.5% during the forecast period of 2024 to 2032. Repeat expansion disorders are a group of inherited conditions that are triggered by expansions in the DNA repeats of affected individuals. The length of the DNA repeats can range from a single nucleotide all the way up to a dodecamer or even longer. The point at which the repetitive expansions start to cause symptoms differs from one disease to another depending on what it is. These expansions in DNA sequence are now known to be the root cause of over 40 different illnesses. The causal mutations that drive pathogenesis in a substantial number of these disorders have been recognised thanks to remarkable progress that has been made over the past three decades. The expansion of CAG, GCG, CTG, CGG, and CAAA repeats both in coding and non-coding regions in separate genes results in a wide set of disorders with processes associated to protein levels or toxicity, RNA, and/or both. These repeats can be found in either coding or non-coding sequences.
Increasing Research of Rapid Expansion Disorders Therapies is Boosting the Market
Because of the complexity of repetitive disease genetics and pathobiology, previously unknown commonalities and molecular mechanisms associated with the diseases, such as RNA toxicity, have been uncovered. The investigation of polyglutamine diseases has also shown that post-translational modification is an important step in the pathogenic cascade, and it has demonstrated that the autophagy pathway plays an important role in the degradation of misfolded proteins. These are two themes that are likely to be applicable to the entire field of neurodegeneration. Research on recurrent diseases is yielding insights that are sparking new research avenues. These new lines of inquiry should not only elucidate the molecular mechanisms of disease, but also highlight opportunities for therapeutic intervention in the treatment of conditions that are currently incurable. Despite of these advancements, there are challenges – just as there were when the science was just starting out – in pinpointing the precise manner in which repetitive expansion mutations lead to inherited disorders. As a result, the classification schemes that are currently in use run the risk of becoming obsolete in the near future. In point of fact, a number of discoveries made relatively recently have shed light on the potential complexity of the molecular pathways underpinning the pathogenesis of a variety of diseases caused by repeat expansions. For instance, extensive research into diseases known as CAG-polyglutamine diseases has shown that post-translational modification of disease proteins is an important step in the progression of the disease, and there is now evidence to suggest that the autophagy pathway plays an important part in the breakdown of misfolded proteins.
Market to Present Significant Opportunities During the Forecast Period
Since the first discovery of a novel and obscure type of mutation as the root cause of fragile X syndrome and spinal muscular atrophy (SBMA), a significant amount of progress has been made. The research that has been done in this area has uncovered an extraordinary unanticipated complexity, which includes toxicities that are mediated at the protein, RNA, and genome levels. Research on recurrent diseases, for instance, continues to be one of the most important factors in determining how faulty RNA metabolism and autophagy contribute to human illness. Why the development of relevant treatments has not kept pace with the extraordinary trajectory of scientific advance in the understanding of mechanisms and pathways is an issue that keeps coming up in the field of recurrent disease. This disconnect applies to the vast majority of neurological disorders; nevertheless, repeat diseases present a fantastic potential for the development of reasonable therapies due to the fact that conclusive diagnosis is typically predicated upon repeat allele expansion. These discoveries have also led to some very encouraging therapeutic developments, such as the utilisation of oligonucleotide and morpholino anti-sense repeats targeted against the DM1 CTG expansion.
Clinical Trial Process for Lack of Patient Pool Presents Complexities in Designing the Therapies
It is anticipated that factors such as difficulties encountered in the process of designing and performing clinical trials for the development of a treatment for rapid expansion disorders treatment market would be among those that will impede the growth of the market during the forecast period. It is challenging to design clinical trials for rare neurodegenerative diseases for instance, Friedreich's ataxia because there are not enough patients with the disease, the disease is heterogeneous, and it has a slow and variable progression rate. These factors make it difficult to identify reliable outcomemeasures to monitor the progression of the disorders.For instance, Friedreich's ataxia is a genetic, neurodegenerative movement disorder, which, according to an article that was published in the Orphanet Journal of Rare Diseases in August 2020, has a typical age of onset (the age at which the symptoms of a disease first appear) that ranges between 10 and 15 years. This age range was determined by taking the average age of patients diagnosed with Friedreich's ataxia. As a result, it takes a significant amount of time from the disease's onset until it can be properly diagnosed.
Huntington Disease Treatment Market Presenting Largest Opportunities
Huntington disease treatment market among other rapid expansion disorders treatment is expected grow the fastest registering a CAGR of 20% during the forecast period 2024 to 2032. It is estimated that important drivers during the forecast period will include the planned label expansion of Ingrezza for the treatment of chorea associated with Huntington's disease, the high burden of HD in western countries, and a robust product pipeline of disease-modifying treatments. There are around 30,000 persons in North America who have HD, and the disease has a prevalence of 5.7 cases per 100,000 people. HD in children is significantly less common than in adults, accounting for only approximately 5–10% of all cases. It affects as many as 10 people out of every 1,00,000 people in Europe.
There is a greater than tenfold difference in prevalence based on geographic location, which may be due to differences in case ascertainment and diagnostic criteria. While the populations of Europe, North America, and Australia have a larger incidence, the prevalence among the Asian population has generally been lower. Targeting HD's hyperactive immune system with immunomodulatory medicines is one of the primary focuses of a number of small molecules now undergoing clinical development.
North America Remains as the Global Leader and APAC to Present the Largest Opportunity
In 2021, North America held the dominant position in the market for treatments for Huntington's disease, holding a revenue share of 60%. This expansion is due to the high burden of the disease, increased healthcare expenditure, technological advancements, proactive government initiatives, and increased patient awareness about treatments available for rapid expansion disorders treatment. Specifically, the high burden of the disease is causing increased healthcare expenditure.Due to increased product penetration, untapped potential, and the growing prevalence of the conditions in emerging nations throughout the projection period, the market for Huntington's disease treatment is expected to exhibit the highest CAGR in Asia Pacific during the forecast period of 2024 to 2032. It is anticipated that an increasing number of measures being implemented by governments to enhance the health status of the population will fuel the expansion of the market in the region. However, there is a lot of competition in the market for treatments for Huntington's disease, which may slow down its expansion in this region.
Intense R&D Focus to Enhance the Market Share of the Market Players
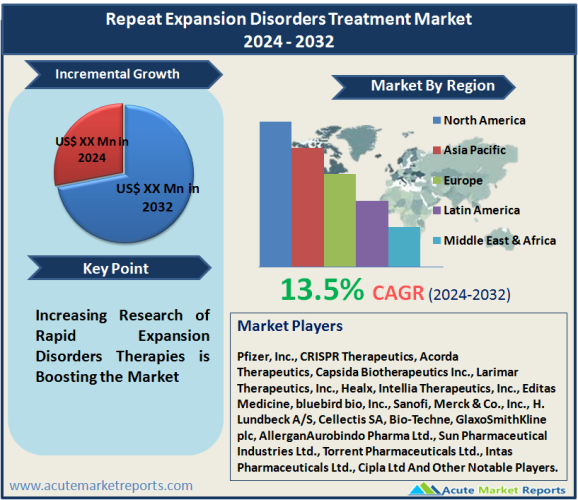
Market is fragmented due to the scope of market that is characterized by different pharma companies leading different therapeutic segment across countries.Therefore, the competition is significantly high and is expected further intensify during the forecast period of 2024 to 2032. The key strategy adopted by Tier 1 companies is focus on R&D to develop novel therapeutic options. Some of the prominent companies dominating the market includes Pfizer, Inc., CRISPR Therapeutics, Acorda Therapeutics, Capsida Biotherapeutics Inc., Larimar Therapeutics, Inc., Healx, Intellia Therapeutics, Inc., Editas Medicine, bluebird bio, Inc., Sanofi, Merck & Co., Inc., H. Lundbeck A/S, Cellectis SA, Bio-Techne, GlaxoSmithKline plc, AllerganAurobindo Pharma Ltd., Sun Pharmaceutical Industries Ltd., Torrent Pharmaceuticals Ltd., Intas Pharmaceuticals Ltd., Cipla Ltd and others.
Historical & Forecast Period
This study report represents analysis of each segment from 2022 to 2032 considering 2023 as the base year. Compounded Annual Growth Rate (CAGR) for each of the respective segments estimated for the forecast period of 2024 to 2032.
The current report comprises of quantitative market estimations for each micro market for every geographical region and qualitative market analysis such as micro and macro environment analysis, market trends, competitive intelligence, segment analysis, porters five force model, top winning strategies, top investment markets, emerging trends and technological analysis, case studies, strategic conclusions and recommendations and other key market insights.
Research Methodology
The complete research study was conducted in three phases, namely: secondary research, primary research, and expert panel review. key data point that enables the estimation of Repeat Expansion Disorders Treatment market are as follows:
Market forecast was performed through proprietary software that analyzes various qualitative and quantitative factors. Growth rate and CAGR were estimated through intensive secondary and primary research. Data triangulation across various data points provides accuracy across various analyzed market segments in the report. Application of both top down and bottom-up approach for validation of market estimation assures logical, methodical and mathematical consistency of the quantitative data.
| ATTRIBUTE | DETAILS |
|---|---|
| Research Period | 2022-2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Year | 2022 |
| Unit | USD Million |
| Segmentation | |
Disorders
| |
Route of Administration
| |
Distribution Channel
| |
Drug Pipeline (Profile)
| |
|
Region Segment (2022-2032; US$ Million)
|
Key questions answered in this report